Quote:
Originally Posted by mrhasan

Hows the dosing done Michael? Because to have one sodium ion for every chloride ion, there has to be a ratio of 110.98g of CaCl for every 84.007g of CaHCO (molar masses). Does that satisfy the aprox 10ppm of calcium consumption every 2dkh of alk drop? Because if that amount of grams are not maintained, there will either more be more sodium ions or more chloride ions with an end result of being imbalance.
|
And here is your answer in probably more detail than you could ever wish for

Hans-Werner does not hold back

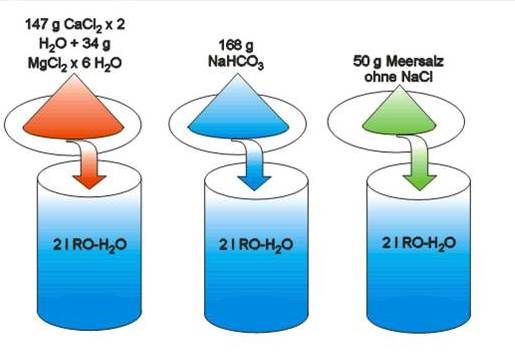
• Sodium chloride has a molar weight of 58.44 g/mol.
• In 2 l of R/O water a max. of 2 mol sodium bicarbonate can be dissolved. After addition of 2 mol sodium bicarbonate and 1 mol calcium chloride 2 mol sodium chloride remain in the aquarium. 2 mol sodium chloride can be balanced with exactly 50 g of sodium chloride free sea salt.
• Formula:
CaCl2 x 2 H2O + 2 NaHCO3
CaCO3 + 2 NaCl + CO2 + 3 H2O
• Insert weights:
147 g CaCl2 x 2 H2O + 168 g NaHCO3 100 g CaCO3 + 117 g NaCl + 44 g CO2 + 54 g H2O
117 g NaCl + 50 g NaCl free sea salt 167 g complete sea salt
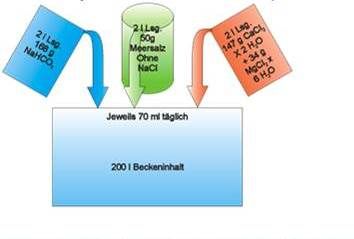
• Adjust tank water to 7° KH and 420 ppm calcium.
• Check alkalinity after two days. Calculate how much alkalinity solution is needed. Add same volume of all three solutions.
• Continue with daily additions of half the volume.
• Adjust added volumes to keep 7° KH alkalinity.